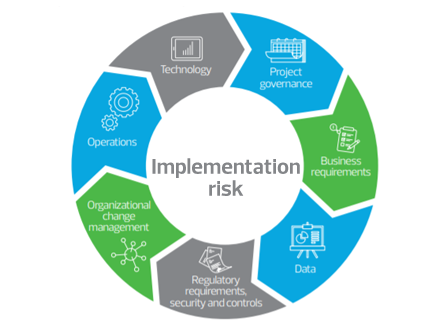
RSM US LLP can help you avoid these pitfalls. Our implementation risk professionals have decades of experience managing program risk; designing regulatory compliant security that also reduces user license costs; managing automated control enablement within various ERPs and applications; and enabling emerging technologies or ERP governance, risk and compliance (GRC) technologies during implementations. Our proven methodology focuses on the risk of key program success factors and is designed to help you realize the full return on your investment.
Our team has deep experience with large custom software implementations, as well as in risk, security and controls with a variety of leading ERP solutions including:
- SAP
- Oracle
- Microsoft Dynamics
- NetSuite
- Workday
RSM not only has deep experience in the configuration of these ERPs with regard to security and automated controls design, but we can also provide risk oversight during your ERP implementation. Whether you need program risk oversight, or an independent verification and validation (IV&V) or the U.S. Food and Drug Administration (FDA) computer system validation (CSV), our knowledge, experience and flexible methodology and approach help us craft a solution tailored to your unique circumstances.